 Found 9 hits of ki data for polymerid = 50004796
Found 9 hits of ki data for polymerid = 50004796 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606675
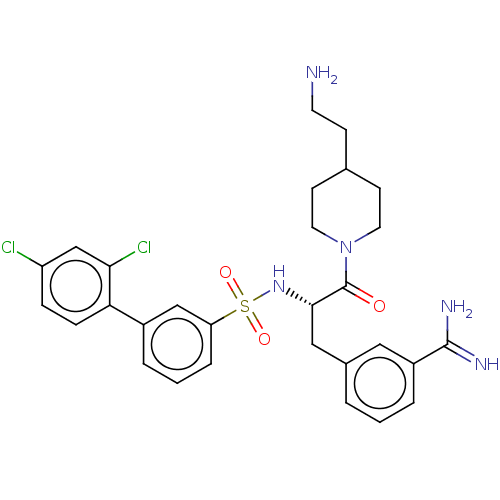
(CHEMBL3219079)Show SMILES NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(Cl)cc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606676
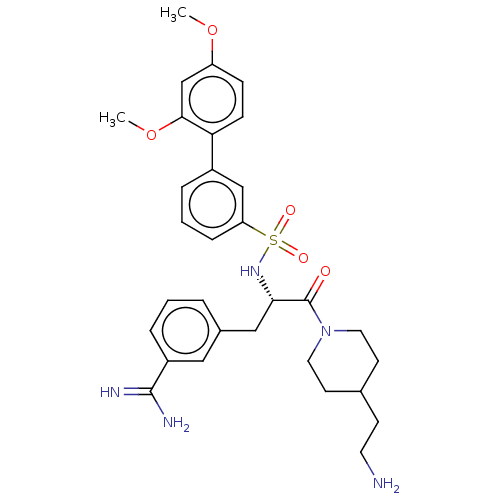
(CHEMBL3219087)Show SMILES COc1ccc(c(OC)c1)-c1cccc(c1)S(=O)(=O)N[C@@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCC(CCN)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606672
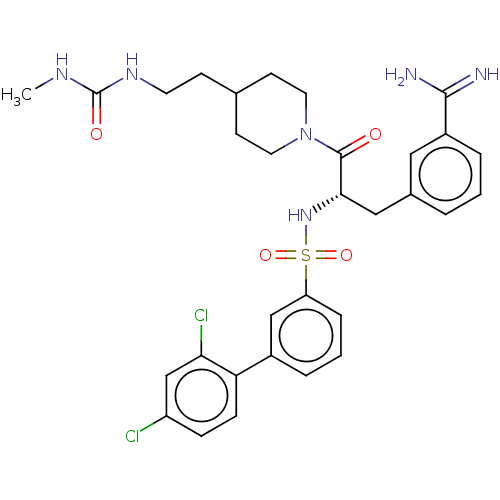
(CHEMBL3219101)Show SMILES CNC(=O)NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(Cl)cc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606674
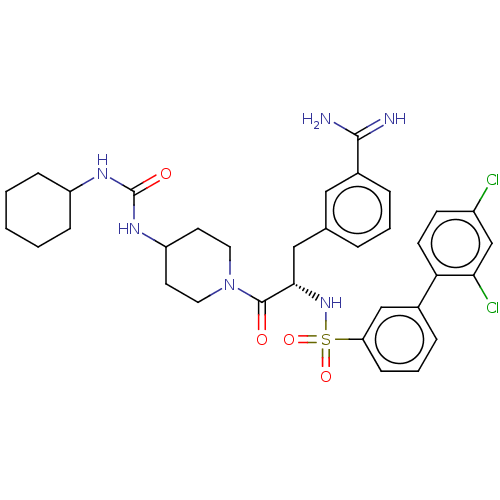
(CHEMBL3219103)Show SMILES NC(=N)c1cccc(C[C@H](NS(=O)(=O)c2cccc(c2)-c2ccc(Cl)cc2Cl)C(=O)N2CCC(CC2)NC(=O)NC2CCCCC2)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606673
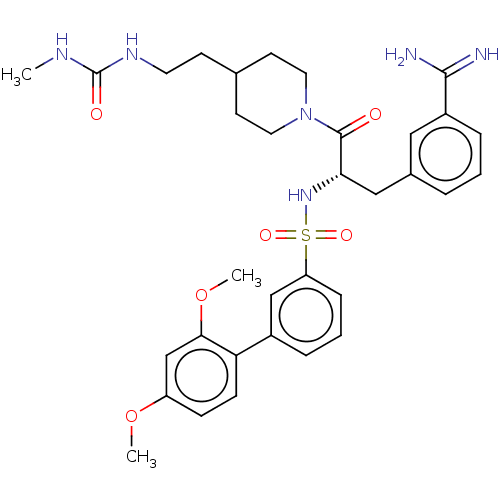
(CHEMBL3219100)Show SMILES CNC(=O)NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(OC)cc1OC |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606671

(CHEMBL3219102)Show SMILES COc1ccc(c(OC)c1)-c1cccc(c1)S(=O)(=O)N[C@@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCC(CC1)NC(=O)NC1CCCCC1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50324475

(Benzylsulfonyl-D-argininyl-proline-(4-amidinobenzy...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H36N8O4S/c27-23(28)20-12-10-18(11-13-20)16-32-24(35)22-9-5-15-34(22)25(36)21(8-4-14-31-26(29)30)33-39(37,38)17-19-6-2-1-3-7-19/h1-3,6-7,10-13,21-22,33H,4-5,8-9,14-17H2,(H3,27,28)(H,32,35)(H4,29,30,31)/t21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50349344

(CHEMBL1809250)Show SMILES [#6]-[#6]-[#6](-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7])-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]S(=O)(=O)[#6]-c1ccccc1 |r| Show InChI InChI=1S/C24H35N7O3S/c1-2-20(30-15-17-10-12-19(13-11-17)23(25)26)22(32)21(9-6-14-29-24(27)28)31-35(33,34)16-18-7-4-3-5-8-18/h3-5,7-8,10-13,20-21,30-31H,2,6,9,14-16H2,1H3,(H3,25,26)(H4,27,28,29)/t20?,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50349345
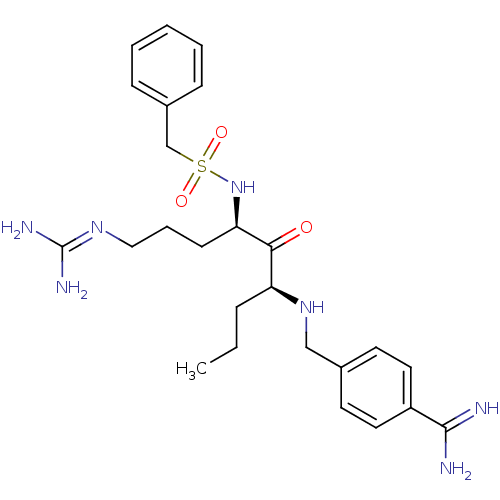
(CHEMBL1809251)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7])-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]S(=O)(=O)[#6]-c1ccccc1 |r| Show InChI InChI=1S/C25H37N7O3S/c1-2-7-21(31-16-18-11-13-20(14-12-18)24(26)27)23(33)22(10-6-15-30-25(28)29)32-36(34,35)17-19-8-4-3-5-9-19/h3-5,8-9,11-14,21-22,31-32H,2,6-7,10,15-17H2,1H3,(H3,26,27)(H4,28,29,30)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data